

What to expect with Elfabrio
An extensively studied enzyme replacement therapy (ERT) for Fabry
There are only about 11,000 people in the United States living with diagnosed Fabry disease. Elfabrio was evaluated in 8 international clinical trials that included 142 people.
The key trials for FDA approval were:
- The BALANCE Study* for people who switched treatments
- The New to ERT Study* for people who hadn’t been treated before or for those not treated at least 6 months before the start of the study
Together, these studies reflect a diverse group of adults with Fabry:

 |
Both males and females |
 |
People with varying levels of kidney function |
 |
People who switched from another ERT |
 |
People who had never been on an ERT before |
| * | In the Elfabrio Full Prescribing Information, the BALANCE Study is referred to as Trial 2, and the New to ERT Study is referred to as Trial 1. |
| FDA, Food and Drug Administration. | |

Elfabrio has been studied in people like you
First time on an ERT?
First time on an ERT?See the results
 |
The BALANCE Study: For people switching treatments |
Elfabrio: Studied against another ERT
The research showed: In BALANCE, our 2-year key clinical trial, a diverse group of adults who had previously been treated with Fabrazyme® (agalsidase beta) were switched over to Elfabrio. Their results with Elfabrio were compared to those of a control group that stayed on Fabrazyme.†
BALANCE study design:
2-year comparison
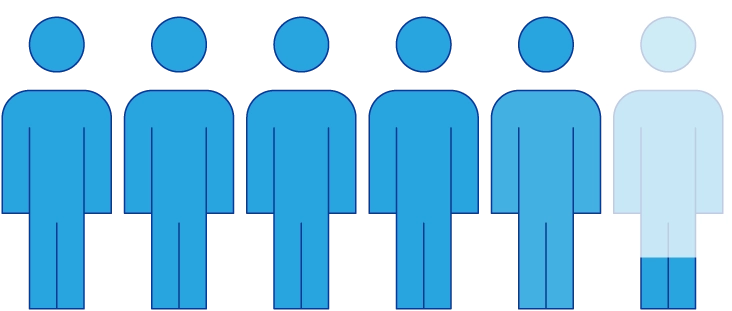
people were switched to Elfabrio from Fabrazyme‡
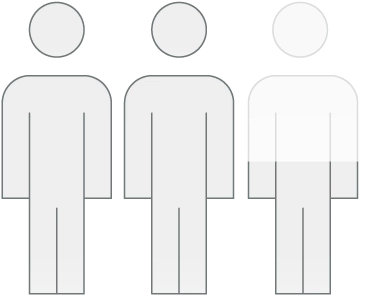
people stayed on
Fabrazyme‡
 = 10 people
= 10 people| † | The data discussed are not intended to establish that Elfabrio is better or is not worse than Fabrazyme. Please see the FDA Approval Package available at Drugs@FDA for more information. |
| ‡ | People were on Fabrazyme for at least 1 year prior to the study and for an average of approximately 6 years. |
Evaluated effectiveness with 2-year data
Some of the ways that Elfabrio was assessed include:
 |
Kidney function |
A primary focus of this study was to assess kidney function for people taking Elfabrio in comparison to those who stayed on Fabrazyme. This was measured by estimated glomerular filtration rate (eGFR) levels, which can help your doctors understand how well your kidneys are working.
The 2-year research showed Elfabrio was proven to work:
Maintained kidney function with Elfabrio
The rate of change in eGFR was similar in people who switched to Elfabrio (–2.4) to those who stayed on Fabrazyme (–2.3). Based on this comparison, both ERTs helped maintain kidney function and slow the progression of disease over the course of the 2-year study.§
 |
Immune response (ADAs) |
Another focus of the study was to understand changes in the immune system during Elfabrio treatment. Researchers tested for anti-drug antibodies (ADAs) at the start of the trial and monitored for changes in ADA tests throughout the study.
The 2-year research showed Elfabrio was proven to work:
Low immune response‖
People who switched to Elfabrio experienced a low rate of newly developed ADAs. 31 out of 34 people (91%) taking Elfabrio who were negative at the start of the study remained ADA negative throughout the study.
Clinical significance of total ADAs and their effect on treatment is not completely understood. Talk to your doctor about testing and monitoring for ADAs during treatment.
In the Elfabrio Full Prescribing Information, the BALANCE Study is referred to as Trial 2.
| § | The FDA has indicated that there were limitations in the BALANCE Study (for example, differences in patient population), such that it could not conclude whether Elfabrio performed the same as or not worse than Fabrazyme. Nonetheless, the FDA stated that the rate of change in eGFR was comparable between the treatments. |
| ‖ | The clinical effect of ADAs on the effectiveness of Elfabrio is not fully understood. The detection of antibody formation depends a lot on the test that is conducted. There was no statistical difference for trends in ADAs between the 2 treatment groups from the beginning to Week 104. |

What are anti-drug antibodies (ADAs)?
ADAs may develop when your immune system reacts to a drug, causing your body to produce antibodies against (“anti”) the drug. ADAs have the potential to affect how well a drug works in your body.
was assessed include:
Elfabrio was proven to work:

Kidney function
A primary focus of this study was to assess kidney function for people taking Elfabrio in comparison to those who stayed on Fabrazyme. This was measured by estimated glomerular filtration rate (eGFR) levels, which can help your doctors understand how well your kidneys are working.
Maintained kidney function with Elfabrio
The rate of change in eGFR was similar in people who switched to Elfabrio (–2.4) to those who stayed on Fabrazyme (–2.3). Based on this comparison, both ERTs helped maintain kidney function and slow the progression of disease over the course of the 2-year study.§

Immune response (ADAs)
Another focus of the study was to understand changes in the immune system during Elfabrio treatment. Researchers tested for anti-drug antibodies (ADAs) at the start of the trial and monitored for changes in ADA tests throughout the study.
Low immune response‖
People who switched to Elfabrio experienced a low rate of newly developed ADAs. 31 out of 34 people (91%) taking Elfabrio who were negative at the start of the study remained ADA negative throughout the study.
Clinical significance of total ADAs and their effect on treatment is not completely understood. Talk to your doctor about testing and monitoring for ADAs during treatment.
| In the Elfabrio Full Prescribing Information, the BALANCE Study is referred to as Trial 2. | |
| § | The FDA has indicated that there were limitations in the BALANCE Study (for example, differences in patient population), such that it could not conclude whether Elfabrio performed the same as or not worse than Fabrazyme. Nonetheless, the FDA stated that the rate of change in eGFR was comparable between the treatments. |
| ‖ | The clinical effect of ADAs on the effectiveness of Elfabrio is not fully understood. The detection of antibody formation depends a lot on the test that is conducted. There was no statistical difference for trends in ADAs between the 2 treatment groups from the beginning to Week 104. |

What are anti-drug antibodies (ADAs)?
ADAs may develop when your immune system reacts to a drug, causing your body to produce antibodies against (“anti”) the drug. ADAs have the potential to affect how well a drug works in your body.
Established safety profile after switching treatments
Elfabrio was well tolerated and the majority of people taking it were able to stay on treatment for 2 years. Side effects and infusion-associated reactions were thoroughly examined. Safety results from the key study are listed below.
The most common adverse reactions that occurred in at least 15% of patients were:
- Infusion-associated reaction
- Common cold
- Headache
- Diarrhea
- Fatigue
- Nausea
- Back pain
- Pain in arms and legs
- Sinus infection
Click here to see the full adverse reaction data from the BALANCE Study.

Low rates of side effects and infusion-related reactions¶#
In this study, when researchers compared the safety results for people who switched to Elfabrio to those who stayed on Fabrazyme:
People taking Elfabrio had at least a
3x lower
rate of side effects
related to treatment
People taking Elfabrio had at least a
7x lower
rate of infusion-related
reactions#
Calculating rates, as shown here, helps researchers understand how likely it is that side effects and infusion-related reactions may happen over time.
Differences in rates of treatment-related side effects and infusion-related reactions were observed in the 2 treatment groups. The ADA-positive group (people who became ADA positive at any time throughout the study) were more likely than people who didn’t develop ADAs to experience side effects and/or infusion-related reactions.
| ADA Positive | ||
| People taking Elfabrio (18 people) |
People taking Fabrazyme (8 people) |
|
| Rate of side effects (considered related to treatment) |
87.18 | 295.80 |
| Rate of infusion- related reactions |
0.9 | 7.5 |
| ADA Negative | ||
| People taking Elfabrio (34 people) |
People taking Fabrazyme (17 people) |
|
| Rate of side effects (considered related to treatment) |
20.08 | 83.64 |
| Rate of infusion- related reactions |
0.3 | 2.2 |
| ADA Positive | ADA Negative | |||
| People taking Elfabrio (18 people) |
People taking Fabrazyme (8 people) |
People taking Elfabrio (34 people) |
People taking Fabrazyme (17 people) |
|
| Rate of side effects (considered related to treatment) |
87.18 | 295.80 | 20.08 | 83.64 |
| Rate of infusion- related reactions |
0.9 | 7.5 | 0.3 | 2.2 |
| ¶ | The data discussed are not intended to establish that Elfabrio is better or is not worse than Fabrazyme on the basis of safety or efficacy. These rates are calculated based on the reported data. Your experience may vary. |
| # | Infusion-related reactions are not injection site reactions. They are side effects related to treatment that occurred during the infusion or within 2 hours after its completion. |
 |
New to ERT Study: For people starting treatment |
Studied over time in people who had never been on Fabry treatment before
The other key trial from the Full Prescribing Information evaluated 18 people who were either new to treatment or hadn’t received ERT in the 6 months before the study. An extension study followed the patients for up to 5 years. The trial assessed the safety of Elfabrio and its effect on kidney tests.
Long-term results
 |
Helped maintain kidney function and slow disease progression, based on results from a 5-year study |
 |
A kidney function test (known as eGFR) showed that both men and women maintained stability over time |
Elfabrio was well tolerated
Assessing treatment safety
In people who had never been on an ERT before or who had stopped treatment for at least 6 months:
- Most side effects were mild or moderate in severity
- 2 patients experienced a severe treatment-related side effect
- 1 person experienced a migraine headache that was possibly related to treatment
- 1 person experienced anaphylaxis related to treatment, which led to withdrawal from the study

Assessing immune system response
In people who were ADA negative at the start of Elfabrio treatment and had ADA-testing results available:
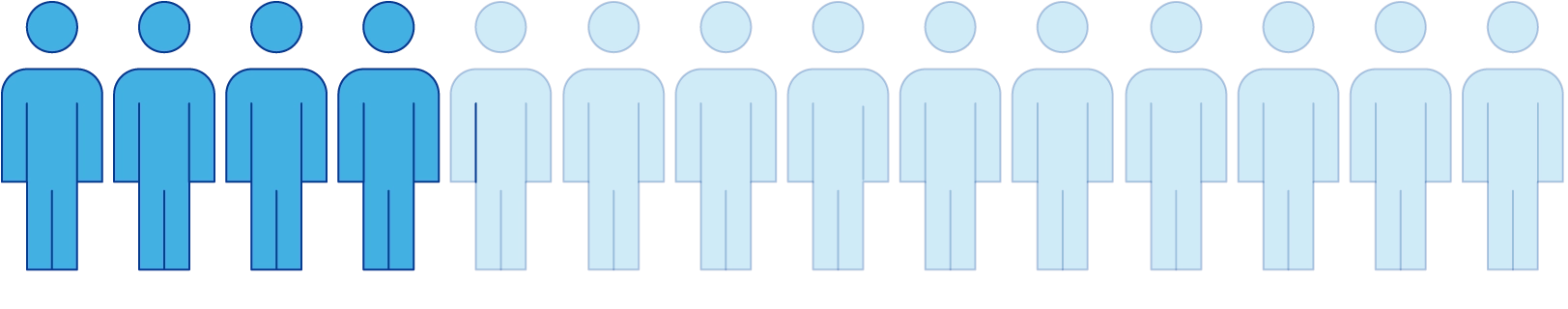
Only 4 out of 14 people (28%) became ADA positive during the 1-year treatment period.
Elfabrio: A treatment you can
start and stay with

Need help talking to your doctor about Elfabrio?
Download the DoctorDiscussion Guide
Managing expectations and side effects
IF YOU NEED IMMEDIATE MEDICAL ASSISTANCE, PLEASE DIAL 911.
If you think you are experiencing a serious side effect with Elfabrio treatment, it is important to call your doctor immediately. In many cases, other side effects are manageable and your doctor will have recommendations. Make a note to discuss it at your next visit. Your doctor may slow your infusion rate as you adjust to treatment with Elfabrio.
Any time you switch treatments or start a new treatment, your body has to get used to it at first. Don’t get discouraged or stop taking Elfabrio unless directed by your doctor. Maintaining a consistent infusion schedule is key to getting consistent results from treatment.
If you have a serious hypersensitivity reaction during the infusion process, the infusion staff or your home health nurse will stop the treatment right away and take appropriate measures.

